Glaucoma
What is glaucoma?
Glaucoma is a large group of progressive eye diseases characterized by damage to the eye’s optic nerve. There is no single, definite cause for glaucoma, but many factors can contribute to the optic nerve damage. Elevated pressure within the eye is one well-known risk factor, but it’s not a cause.
This is because glaucoma can develop at normal eye pressure as well.
The optic nerve is a bundle of millions of nerve fibers at the very back of the eye. It carries all the visual information gathered by other cells in the eye to the brain, where that information is interpreted as images.
Though glaucoma is the most common type of progressive optic nerve damage, it is not the only disease that can damage the optic nerve.
Definition and overview
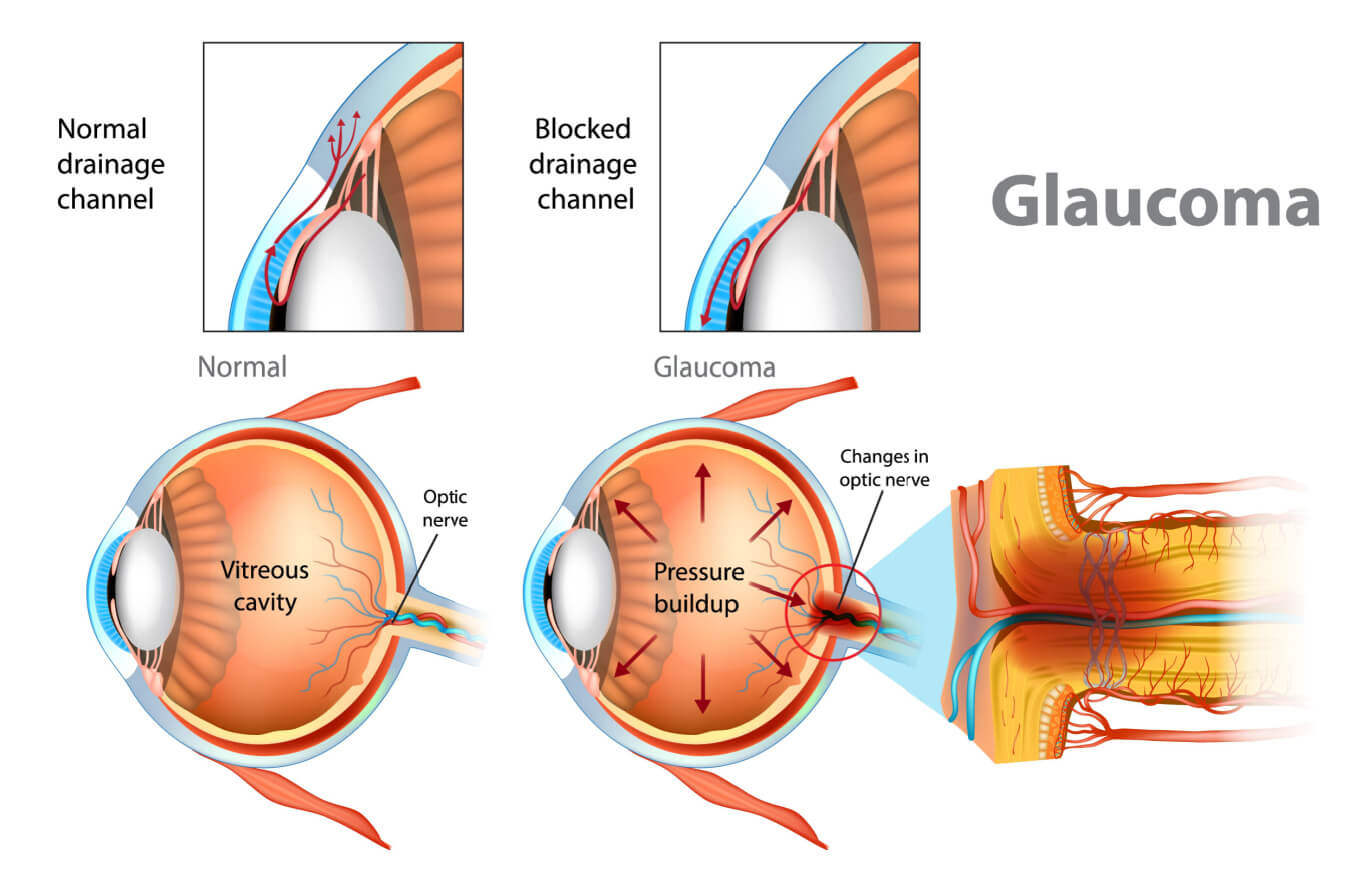
Glaucoma is optic nerve damage caused specifically by pressure inside the eye putting too much outward stress on the eye’s walls and optic disc. The optic disc is the area where the optic nerve fibers leave the back of the eye to connect to the brain.
Too much pressure on the cells in this area can damage or even kill them. These cells can’t ever heal or grow back, so the vision loss is permanent.
This is why high intraocular pressure (IOP) is an important risk factor. Many forms of glaucoma develop because something causes intraocular pressure to rise too much for too long.
Many cases, though, develop despite perfectly typical, average internal eye pressure. And many people never develop the condition despite having long-term elevated IOP. Experts theorize that other aspects of the eye’s structure, like size and wall thickness, may account for how the optic nerve handles stress in those cases.
Optic nerve damage that is caused by eye pressure is called glaucomatous damage. It is visible during an eye exam as a distinctive cupping out of the optic disc.
Eye doctors have extensive training in recognizing the different types of optic nerve damage. When they detect glaucomatous damage during an eye exam, they diagnose it as glaucoma.
LEARN MORE about what causes glaucoma
What is intraocular pressure (IOP)?
IOP is the amount of outward stress exerted by the fluids inside the eye on its internal walls. This stress, or pressure, is very important in maintaining the shape and proper functioning of the eye.
Our eyes are filled with two types of fluid: vitreous humor and aqueous humor. Both of these fluids provide nutrients to the eye, among other important jobs.
Vitreous humor fills the vitreous cavity, which is the space in the eye between the back of the lens and the retina. It is responsible for the majority of the eye’s volume and shape, and it also helps support the retina.
The aqueous humor fills the anterior chamber, which is the space between the cornea and the front of the iris. Unlike vitreous humor, new aqueous humor is constantly being made by the eye. This fluid flows into and out of the front portion of the eye, continuously refreshing itself.
Because it’s always flowing, aqueous humor plays a critical role in maintaining the eye’s ideal pressure. If anything slows its ability to drain, eye pressure can rise. When eye doctors measure IOP, they are measuring aqueous humor pressure.
LEARN MORE about intraocular pressure
Different types of glaucoma
There are four main types of glaucoma and also several subtypes. Most of them develop because something slows down or blocks the aqueous humor from draining out of the anterior chamber properly. The four main types of glaucoma are loosely categorized by the location and cause of the drainage problem:
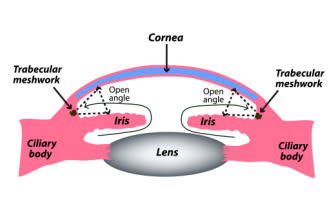
Open-angle – The drainage angle appears normal and open. Outflow problems are present on a microscopic level farther along in the drainage pathway.
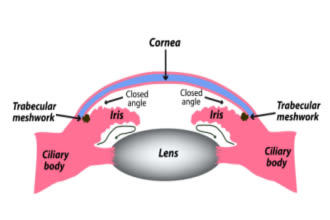
Narrow-angle (also called angle-closure) – The drainage angle itself is narrow or closed, causing outflow problems.
Normal tension – There is no known outflow issue, and IOP is within normal range.
Secondary – This type can be open-angle or narrow-angle, but the drainage problem develops as a result of a condition or injury that was present first.
So, to understand the different types of glaucoma, it helps to learn a little more about how the aqueous humor flows through the anterior segment.
The anterior segment consists of the cornea, iris, pupil, lens and ciliary body. The space inside the anterior segment between the cornea and the lens is called the anterior chamber.
The ciliary body is a ring of tissue and muscle that is connected to the back side of the iris and the outer edges of the eye’s lens. It adjusts the shape of the lens for focusing, and it also produces all of the eye’s aqueous humor.
This fluid is released from the ciliary body and follows a path between the lens and iris and then out into the anterior chamber through the pupil. It then leaves the anterior chamber through an area called the drainage angle. This area is located at the junction where the iris and cornea
The drainage angle funnels the fluid into another structure called the trabecular meshwork (TM). The TM is a complex tissue that filters the fluid leaving the eye. From there, the fluid moves through Schlemm’s canal, another part of the drainage pathway, before being reabsorbed by the body.
There is also a second drainage route called the uveoscleral outflow pathway. This pathway bypasses the TM, and fluid drains directly through the wall of the eye at the base of the iris and ciliary body.
When something along these pathways interferes with the fluid leaving the eye, it causes intraocular pressure to rise. This often leads to glaucoma.
If the drainage angle is open and normal, this is called open-angle glaucoma. If it is blocked or restricted, this is called narrow-angle glaucoma. Both of these types can be either primary or secondary.
Primary glaucomas
Glaucomas are considered primary when no underlying condition or injury can be found as their cause.
For example, if high IOP has caused stress damage to the optic nerve, but there is no identifiable cause for the high IOP, that is a primary glaucoma. If IOP is high due to drainage angle impairment, but the cause of that impairment can’t be identified, that is also a primary glaucoma.
And, if there is stress damage to the optic nerve in an eye that has normal IOP, that is also a primary glaucoma.
Open-angle and narrow-angle forms can both be primary or secondary. However, the two most common types of glaucoma are both primary.
Open-angle glaucoma
Primary open-angle glaucoma (POAG) is by far the most common form of the disease. In the US, 70% to 95% of all glaucoma cases are POAG.
In POAG, the drainage angle that leads to the TM appears fine, but aqueous fluid exits the eye too slowly. POAG is a chronic disease that progresses very slowly. It is painless, and it rarely has any symptoms before its advanced stages.
Angle-closure glaucoma (also called narrow-angle)
Chronic primary angle-closure glaucoma (PACG) is the second most common form of the disease, but it’s much less common than POAG. Only about 10% of US glaucoma cases are angle-closure. Even so, it accounts for about half of all blindness from glaucoma.
Also called narrow-angle glaucoma, PACG usually occurs because pressure behind the iris has caused it to bow forward.
This bowing restricts aqueous flow at both the pupil (called pupillary block) and at the drainage angle. As more fluid collects behind the iris, the bowing becomes more severe.
PACG can also be related to the natural placement and/or size of the iris or ciliary body. In some eyes, the iris is thicker or sits slightly more forward in the angle. In others, the ciliary body is too large for the eye or too close to the back of the iris.
These anatomical traits, known as plateau iris, cause crowding of the space and naturally narrow angles.
PACG can be either chronic (the angle narrows slowly or intermittently) or acute (the angle narrows or closes suddenly). Acute angle-closure is very rare. Only about 30% of people with PACG ever experience an acute attack. Acute angle-closure can also be due to injury or other secondary causes.
Acute angle-closure is a medical emergency. It can result in blindness in only one or two days and so requires immediate medical treatment.
Normal-tension glaucoma
In normal-tension glaucoma (NTG), also called low-tension glaucoma (LTG), optic nerve damage and vision loss occur even though IOP is within the average range. There is no known outflow issue, and IOP is within normal range. NTG is usually labeled as a subtype of open-angle glaucoma and is quite common.
Experts have several theories about how NTG develops without elevated IOP. For example, it may be related to poor blood supply to the optic nerve, or the optic nerve itself may be extra sensitive to normal pressure. It’s also possible that eye diameter, wall thickness and corneal thickness influence how pressure affects the optic nerve.
Congenital glaucoma
Childhood glaucomas are rare in the US, occurring in only around 0.0023% of children. There are two primary forms: primary congenital glaucoma (PCG) and juvenile open-angle glaucoma (JOAG). PCG accounts for 50% to 70% of all childhood glaucomas. There are also several secondary forms.
PCG can be present at birth, but the age of onset is often between 3-9 months. Unlike most other types of glaucoma, PCG is aggressive and can cause severe damage throughout the eye.
Babies with PCG have developmental abnormalities in their eyes’ drainage systems. The abnormalities lead to fluid buildup and higher IOP. Because babies' eyes are still so soft and malleable, the extra fluid also causes the eyes to stretch and grow too large.
Enlarged eyeballs, called buphthalmos, is a common sign of PCG. This stretching is what can cause types of eye damage not seen in adult glaucoma.
LEARN MORE about childhood glaucomas
Secondary glaucoma
Glaucomas are considered secondary when they develop due to known causes.
For example, eye injuries or side effects of certain medications may lead to high IOP and glaucoma. Underlying medical conditions, including some eye conditions, can also contribute to glaucomatous optic nerve damage. Several types of syndromes are also associated with the condition.
There are many more secondary types of glaucoma than primary, but they only account for an estimated 10% of all diagnosed glaucoma worldwide.
Neovascular glaucoma
Neovascular glaucoma (NVG) develops due to the growth of new blood vessels on the iris.
These new blood vessels can eventually have large enough numbers to block the eye’s drainage angle. They are also weaker than typical, healthy blood vessels. This means they tend to break and leak, clogging the drainage system with blood.
The most common cause for neovascularization of the iris is lack of blood flow to the retina. In most cases, this poor blood flow is due to diabetic retinopathy or central retinal vein occlusion (CRVO).
Pigmentary glaucoma & pigment dispersion syndrome (PDS)
In some eyes, the size and/or placement of the iris can cause it to rub backward against the lens. When this happens, the friction can displace tiny granules of the iris’s pigment. These granules then make their way into the very front of the eye. This is called pigment dispersion syndrome (PDS).
These pigmentary granules can eventually clog or block the drainage angle and cause IOP to rise. If the blockage leads to optic nerve damage, that is pigmentary glaucoma (PG).
PG only accounts for about 1.5% of all glaucoma, but both PDS and PG tend to be more common among white males who have myopia (nearsightedness).
Exfoliative glaucoma
This form is similar to PG in many ways. Exfoliative glaucoma (EG), often referred to as pseudoexfoliative glaucoma (PXG), develops due to exfoliative syndrome. This is a systemic syndrome that causes the body to have a type of internal dandruff.
Tiny flakes of cellular debris accumulate in tissues and organs throughout the body, including the eyes. If enough of this material collects around the drainage angle, IOP can rise and lead to optic nerve damage. This is called exfoliative glaucoma or pseudoexfoliative glaucoma.
PXG is the most common type of secondary glaucoma.
Uveitic glaucoma
This form develops secondarily to uveitis, which is inflammation of the uvea. The uvea is the middle layer of the eye’s wall and, at the front of the eye, also grows away from the wall to form the ciliary body and the iris.
There are a few different types of uveitis and several different things that may cause it. The inflammation can affect the entire anterior segment and impact aqueous flow in multiple ways at once.
Depending on which structures are affected, uveitic glaucoma can be open- or closed-angle, and it can be chronic or acute. It is considered one of the most challenging forms to treat. If someone has symptoms of uveitis, they should see an eye doctor right away.
Traumatic glaucoma
When an eye suffers any type of injury or trauma, the inflammation and/or damage to its tissues and internal structures will often lead to elevated IOP. An estimated 3% to 10% of people who experience eye trauma eventually develop traumatic glaucoma.
The injuries are most often associated with sports, assault, falls and jobsite accidents. However, in rare cases, this form can also develop after intraocular surgery, such as cataract surgery. This is due to an autoimmune reaction that some patients experience after the surgery.
Traumatic glaucoma can be early onset or late onset. In many cases, it can take months and even years before the damage inside the eye leads to glaucoma.
Angle-recession glaucoma is a late-onset form caused by injury. A blow to the eye can displace the aqueous fluid with enough force to push the drainage angle backward. The force can also tear tissues in the trabecular meshwork and ciliary body.
Over time, these tears form scars that slow down outflow and raise IOP.
Topiramate glaucoma
Topiramate is a medication that treats migraines, seizures and neuropathic pain. In some people, it can cause secondary angle closure. Fortunately, this is rare, occurring in only three out of 100,000 people taking the medication.
Topiramate glaucoma tends to affect both eyes, unlike PACG, which usually only occurs in one eye. Eye pressure usually rises in both eyes within the first weeks of taking topiramate.
Steroid-induced glaucoma
Doctors prescribe corticosteroids for the management of pain, inflammation and swelling. They can be taken in many forms, such as pills, injections, nasal sprays, and eye drops or ointments.
All forms of steroids increase the risk of an increase in IOP. But some types of steroids and certain routes of application have a higher risk than others. When the IOP goes up after taking steroid medications, this is called a steroid response.
People at increased risk of steroid-induced glaucoma include those who:
Have glaucoma already
Have a family history of glaucoma
Have Type 1 diabetes
Have high myopia
Are under 6 years old
Have certain connective tissue diseases
Have had a penetrating keratoplasty (corneal replacement surgery)
LEARN MORE about types of secondary glaucoma
Recognizing the symptoms
Most forms of glaucoma rarely have any early symptoms. This includes primary open-angle glaucoma (POAG), the most common form.
The damage to the optic nerve usually happens very slowly, over the course of years. Further, a large portion of the nerve fibers (around 30%) have to be damaged before the vision loss is noticeable. This is why glaucoma is known as the “silent thief.”
When vision loss does become noticeable, it is typically peripheral vision that is affected first. The only way to detect POAG and most other types of glaucoma before this occurs is with yearly comprehensive eye exams.
However, there are forms of glaucoma that do commonly have early symptoms or signs.
Acute angle-closure glaucoma comes on very quickly and can cause sudden, intense eye pain and headaches.
It may also cause:
Nausea
Vomiting
Blurry vision
Rainbow colored halo around lights
If you think you have acute angle-closure glaucoma, get emergency treatment right away. This form of the condition can cause blindness within days.
Congenital glaucoma can often be detected by visible signs. This form is present at birth or has its onset within a few months of birth.
Newborns and infants with congenital glaucoma often have eyes that are noticeably larger than normal and that appear hazy or cloudy. Other possible signs include:
It is possible for all types of glaucoma to cause blurred vision, headaches and haloes when eye pressure is high enough. However, this is rare.
It’s important to note that, even though these forms can be noticeable right away, their symptoms and signs are not truly “early.” They indicate that the optic nerve damage is already well underway, and treatment is needed immediately.
It’s important to have eye exams on a regular basis, even if you aren’t at a higher risk for glaucoma. The American Optometric Association recommends yearly comprehensive eye exams for everyone beginning at age 6. These exams check for all types of eye disease, and they can also often detect other types of health conditions.
However, it’s especially important for people with a higher risk for glaucoma to have regular eye exams. The condition does not usually have any symptoms until there is significant vision loss. So it’s important not to wait for vision symptoms to see your eye doctor.
Understanding the causes
Anyone can develop glaucoma — 80 million people, from newborns to seniors, have the condition globally. Currently, there is no cure for glaucoma or any proven way to prevent it. However, we do know of many major risk factors.
Understanding your risk factors can help ensure that you’re having eye exams often enough to detect it early — before there is extensive vision loss. With early enough detection, it is possible to slow or prevent further damage to the optic nerve.
Those who are at a higher risk of developing glaucoma include:
People who have high IOP
People over age 40
People who have a family history of glaucoma
People with African, Asian or Latin American ancestry
People with certain medical conditions, such as diabetes and high blood pressure
People who use certain medications, specifically steroids
People who have had eye injuries or eye surgery
People who have thin corneas
People who have had other eye conditions, including infections, inflammation, tumors or retinal detachment
People who have myopia (nearsightedness)
People who have hyperopia (farsightedness)
The key to preventing vision loss from glaucoma is getting yearly comprehensive eye exams, even if your vision seems normal. When your eye doctor suspects glaucoma or has initiated treatment, they may ask you to come in more often.
LEARN MORE about potential ways to delay or prevent glaucoma
How glaucoma is diagnosed
Glaucoma can only be diagnosed through a series of tests performed by an ophthalmologist or optometrist. The tests include many of the same procedures that are part of a typical comprehensive eye exam, plus a few that are more specialized.
Like with any kind of medical testing, they will ask you questions about your health history first. Then, depending on the order they perform the tests, they may dilate your pupils or numb your eyes with eye drops.
Pupil dilation is required for some of the tests, but numbing drops are not always necessary. Glaucoma tests are non-invasive and painless. However, the numbing drops can make it easier for you to avoid blinking when the doctor touches your eyes.
Comprehensive eye exam
Because glaucoma rarely has noticeable symptoms, the key to detection and diagnosis is having comprehensive eye exams on a regular basis. These exams allow your eye doctor to monitor your overall eye health and perform further testing if they see any signs of eye disease, including signs of glaucoma.
Your eye doctor may also perform glaucoma tests as part of every eye exam if you are considered a glaucoma suspect. This means that something in previous eye exams or in your medical history shows that you have a higher risk for developing the condition.
In some cases, eye doctors will do these tests if patients experience certain vision symptoms, such as blind spots, tunnel vision or colorful halos around lights.
The doctor can usually discuss your results with you right away. If your results show additional signs of glaucoma, they may recommend treatment options and/or more frequent exams to watch for progression.
Perimetry test
Perimetry, or visual field testing, tells your eye doctor if your visual field has been affected. Vision loss in glaucoma typically begins with peripheral vision. This test will check your peripheral vision, but it can also determine if central vision is affected.
There are two types of perimetry: screening and threshold. During screening perimetry, the machine presents spots of light of only one level of brightness. This test is used to detect whether peripheral vision is normal or abnormal.
During threshold perimetry, the machine shows lights of different intensities. Threshold perimetry measures the degree of peripheral field loss. It takes longer to do this test, but it is the best way to follow glaucoma progression.
The visual field is often normal in the early phase of glaucoma, so perimetry may not need to be done as often.
Tonometry
This test is used to measure your intraocular pressure (IOP). There are three basic types of tonometry tests: applanation, non-contact and electronic indentation tonometry.
In applanation tonometry, you rest your chin and forehead on the familiar slit lamp microscope. The doctor watches through the slit lamp as a very small device briefly touches your cornea to take the measurement. Applanation tonometry is considered the most accurate form of IOP testing.
In non-contact tonometry, also called NCT or the “air puff” test, no device touches the eye. You face an instrument that emits a gentle puff of air to the surface of your eye. The puff of air flattens the center of the cornea briefly to measure eye pressure. NCT is not the most accurate measurement but can be useful in some circumstances.
In electronic contact tonometry, the doctor uses a device that looks like a pen to gently apply pressure to the cornea. The pen generates a reading that displays on a screen. There are several versions of this type of tonometry that vary slightly.
Pachymetry
This test measures the thickness of the cornea, which is important in screening for glaucoma for two reasons.
One reason is that thinner corneal tissue is a known risk factor for developing glaucoma. The other reason is that the thickness or thinness of corneal tissue can impact how accurate the IOP measurement is. There are two common methods of pachymetry testing: ultrasonic and optical.
In ultrasonic pachymetry, the doctor touches the cornea with a small probe that measures its thickness using ultrasonic waves. In optical pachymetry, nothing touches the eye. The doctor uses a special attachment for the slit lamp microscope that can measure corneal thickness with split-image comparisons of the cornea’s endothelium.
Gonioscopy
This test allows the eye doctor to view the drainage angle in your eye.
It is tiny and in a difficult spot to view, so the eye doctor uses a special device called a goniolens. The goniolens is shaped somewhat like a contact lens and fits lightly over the cornea. It contains prisms that work like mirrors to reflect a view of the hidden angle.
The doctor uses the magnification of the slit lamp in combination with the goniolens to see if the angle is open or closed. They will examine the angle for things that could be blocking it, such as scarring, angle recession and plateau iris. They will also check for things that could be clogging it, such as pigment, pseudoexfoliation debris and new blood vessels (neovascularization).
Fundoscopy
A fundoscopic exam is a dilated eye exam that lets the doctor look at the retina and the optic nerve at the very back of your eye.
The doctor may use the slit lamp or a special hand-held tool, or a combination of the two, to get a magnified view of these structures. This part of the test is critical in determining whether the optic nerve and retinal cells have been damaged.
Optical coherence tomography (OCT)
As part of the eye exam, the eye doctor may also take some scans or images of the optic nerve. Optic nerve imaging can be extremely useful in monitoring for any issues.
Stereoscopic photos of the optic disc allow the doctor to monitor the appearance of the optic nerve. They provide a view of the depth and size of the cupping in the center of the optic disc. Stereoscopic photos were standard in the past, but they have largely been replaced by optical coherence tomography (OCT) imaging.
OCT imaging is an accurate way to evaluate the thickness of different layers of the retina. The two most important layers to monitor for glaucoma are the retinal nerve fiber layer (RNFL) and the ganglion cell complex (GCC).
Checking the RNFL for thinning in an area close to the optic nerve is a very sensitive way to detect early glaucoma. Images of the GCC, which is in the center of the retina (the macula), can be compared with images of the RNFL as a very accurate way to monitor progression over time.
Treatment options
Medical treatments for glaucoma focus on lowering the patient’s IOP. Even though IOP isn’t a cause of glaucoma, it is the risk factor that doctors can modify most successfully.
In most cases, lowering IOP can slow or stop the progressive damage being done to the optic nerve. However, any damage that is already done is permanent. There is, unfortunately, no treatment that can reverse or cure optic nerve damage.
While the treatments all aim to lower IOP, the specific treatments that are best for each patient will depend on the type of glaucoma they have and how advanced it is. Other factors that determine treatment options include prior eye surgeries and how much the IOP needs to be lowered.
There are three main categories of treatments: medications, laser procedures and surgery.
Medications
The first line of treatment is typically prescription eye drops. Oral medications are also used in some cases, but this is less common. There are several types of eye drops to treat glaucoma, and they each work in different ways to reduce IOP.
Some increase the rate that aqueous humor can flow out of the eye, and others decrease the amount of aqueous humor made by the ciliary body. Some of them produce a combination of both effects. It’s also fairly common for eye doctors to prescribe a combination of different eye drops.
Prostaglandin analogs work by increasing uveoscleral outflow.
Prostaglandin analogs – Latanoprost, travoprost, bimatoprost and tafluprost
Beta blockers, alpha adrenergic agonists and carbonic anhydrase inhibitors work by reducing the amount of aqueous humor the ciliary body produces.
Beta blockers – Timolol and levobunolol
Alpha adrenergic agonists – Brimonidine and apraclonidine
Carbonic anhydrase inhibitors – Dorzolamide, acetazolamide and methazolamide
Cholinergic agents work by increasing aqueous outflow through the trabecular meshwork.
Cholinergic agents – Pilocarpine
Nitric oxide donors can reduce aqueous humor production as well as increase outflow via the trabecular meshwork.
Nitric oxide donors – Latanoprostene bunod
Prostaglandin EP2 agonists can increase both uveoscleral and trabecular meshwork outflow.
Prostaglandin EP2 agonists – Omidenepag isopropyl
Rho kinase inhibitors can reduce IOP in all three ways. Along with alpha adrenergic agonists, they may also be able to strengthen the optic nerve against glaucomatous damage.
Rho kinase inhibitors – Netarsudil mesylate
It is critical to use glaucoma eye drops consistently and according to your doctor’s instructions. They are not effective if not used consistently. If you notice any negative side effects, talk to your doctor about trying other medications rather than using the eye drops less often or incorrectly.
Marijuana
The American Academy of Ophthalmology, the American Glaucoma Society and the Canadian Ophthalmological Society all agree that people who have glaucoma should not use marijuana to treat the condition.
Marijuana can lower IOP, but it also lowers blood pressure. This means the optic nerve, which is already damaged, receives less blood flow for several hours after THC enters the body. The dangers of reducing blood supply to the optic nerve far outweigh any benefit marijuana can have on IOP.
LEARN MORE about glaucoma medications
Laser treatment
Like the eye drops, laser surgeries lower IOP by improving drainage or by reducing aqueous humor production. They are usually the preferred alternative treatment if eye drops haven’t lowered IOP enough.
They can also be a good option for patients who experience side effects from their eye drops or who are unable to use them consistently.
In some cases, doctors may even recommend laser surgery ahead of or in combination with eye drops. A recent large study showed that laser procedures can be an effective first-line treatment.
The effects of most laser procedures only last a few years, so they may need to be performed more than once. However, for many patients, these in-office procedures can delay or even prevent the need for more invasive types of surgery later on.
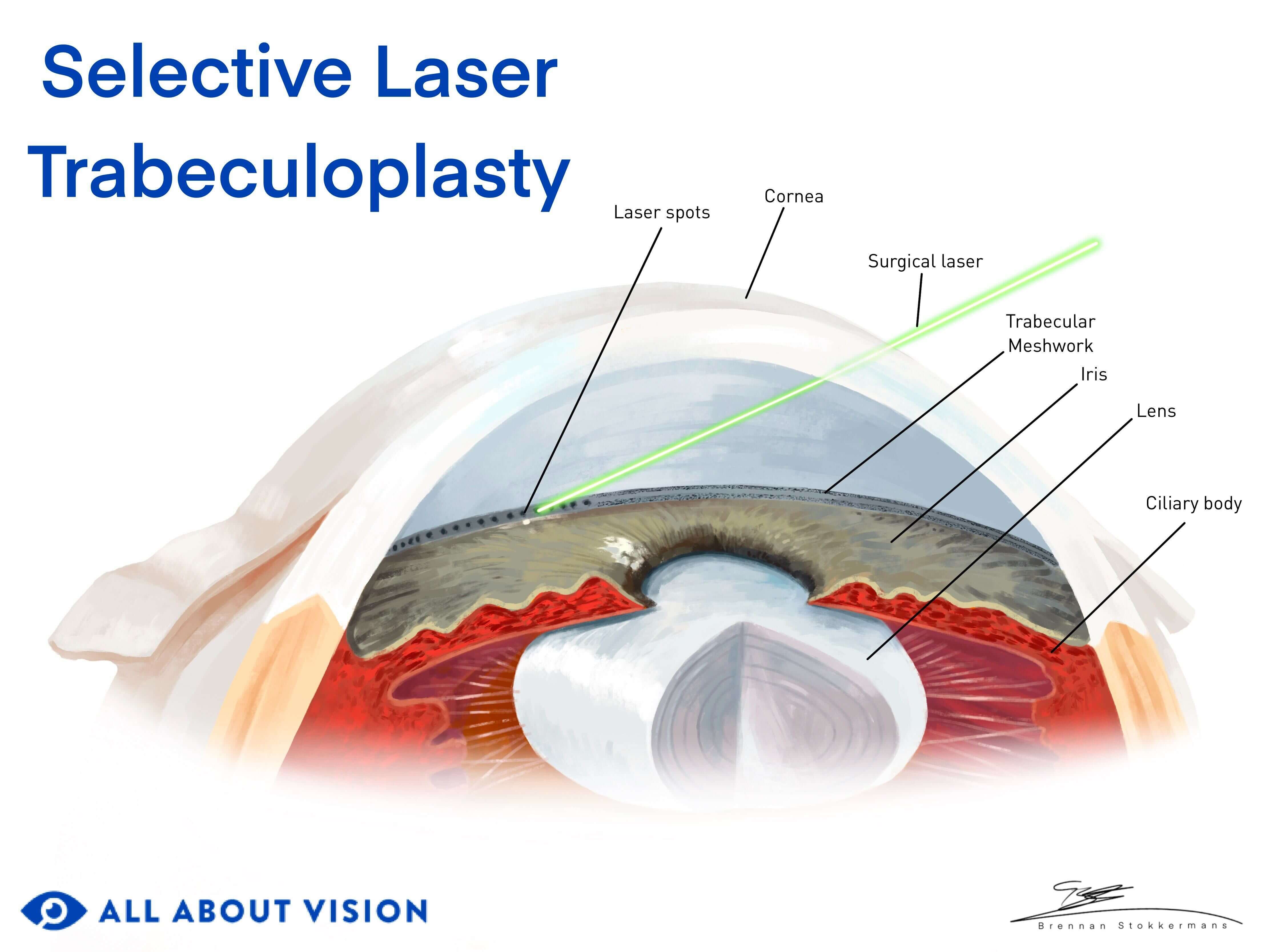
Click on illustration to enlarge.
Trabeculoplasty – Laser trabeculoplasties are often used to treat POAG. They lower IOP by creating openings in clogged trabecular meshwork tissue. The most common forms are selective laser trabeculoplasty (SLT) and argon laser trabeculoplasty (ALT).
Cyclophotocoagulation (CPC) – This procedure uses laser energy to shrink the tissues of the ciliary body and reduce its production of aqueous humor.

Click on illustration to enlarge.
Laser peripheral iridotomy (LPI) – Iridotomies are primarily done to treat closed-angle glaucoma. They can also help to prevent angle-closure in patients who have narrow drainage angles. LPI improves aqueous outflow by creating a tiny hole in the iris so fluid can bypass the pupillary block.
Scatter panretinal photocoagulation – For patients who have neovascular glaucoma, this treatment removes the stimulus that causes abnormal blood vessels to form. It also causes existing abnormal blood vessels to shrink and disappear.
Surgery
Microsurgery (also called filtering or incisional surgery) procedures are usually the next step if eye drops and laser surgery haven’t worked to lower IOP enough. In different ways, they each improve IOP by surgically modifying how fluid leaves the eye.
They are more invasive than laser treatments and have longer recovery periods because they involve incisions. However, the incisions are tiny. The procedures are called microsurgery because eye doctors perform them using microscopes and incredibly small surgical tools.
The benefits of microsurgery can last up to several years, but they aren’t permanent. Fortunately, it is possible to repeat most of them when necessary.

Click on illustration to enlarge.
Trabeculectomy – This procedure creates a small flap, or filtering pocket, under the eyelid in the sclera (the white part of the eye). A tiny piece of the trabecular meshwork beneath the filter is also removed. Aqueous fluid can then bypass the clogged drainage structures and leave the eye through this path. The pocket also keeps fluid from draining too quickly. Fluid accumulates in the filter, forming a small bubble under the conjunctiva called a bleb.
Canaloplasty – This procedure works by dilating Schlemm’s canal (SC) with a microcatheter. The SC is the part of the drainage pathway that aqueous fluid flows into via the trabecular meshwork.
MIGS (minimally invasive glaucoma surgery) – MIGS procedures can be thought of as mini incisional microsurgeries. They are less invasive because the incisions are made in the cornea rather than in the sclera. There are several types of MIGS that lower IOP with various methods. Many of them involve placing very small stents or shunts inside the angle of the eye. They are good options for patients with early or mild glaucoma, but some of them can only be performed in combination with cataract surgery.
Drainage implants – These small implants allow aqueous fluid to leave the eye through tubes or shunts. The base, or plate, of the implant is embedded between the conjunctiva and sclera to hold it in place. The tube or shunt extends from the plate into the anterior chamber. From there, the fluid flows back through the tube into the space between the sclera and the conjunctiva to be reabsorbed by the body.
Peripheral iridectomy – This procedure treats closed-angle glaucoma and involves the removal of a small portion of the edge of the iris. It works in the same way as an LPI to release pupillary block.
Biodegradable drug-delivery implant (Durysta) – Durysta is not technically a surgery, though the implant is injected into the eye with a needle. Once in the eye, the tiny capsule rests in the drainage angle and releases a steady, constant dose of glaucoma medication for 90 days. After the capsule dissolves, the effects can last up to 24 months for some patients. However, the treatment can only be done once per eye.
LEARN MORE about glaucoma surgery
Managing life with glaucoma
While the damage caused by glaucoma is irreversible, timely treatment can help prevent its progression and preserve your remaining vision. There are also steps you can take to preserve your eye health and help make living with glaucoma a little easier, including:
Take or use medications as prescribed by your eye care provider.
Visit your eye doctor as recommended for routine checkups.
Ensure your living areas are well-lit to avoid bumping into objects and potential falls or accidents.
Organize furniture and everyday essentials to make navigating your environment as seamless as possible.
Use magnifying devices or other low vision tools to assist you in reading books or using digital devices.
Get regular exercise to help support eye health, but be sure to consult your doctor first. Certain activities could increase your eye pressure and potentially worsen the condition.
Lean on family and friends for assistance with transportation and other aspects of daily life.
Organizations like the National Eye Institute provide helpful Glaucoma Resources for patients and caregivers. Your eye doctor is also a great resource for identifying additional sources of glaucoma support.
Ongoing research and clinical trials
Ongoing research is focused on advancing glaucoma treatments, including improvements in medication, surgical techniques, and strategies for preventing vision loss.
Researchers are also studying regenerative therapies aimed at protecting retinal ganglion cells, a type of neuron found in the optic nerve. The treatment focuses on promoting cell regeneration and preventing cell death. This offers the potential to slow or even halt glaucoma progression and preserve vision. There are many clinical studies focused on advancing glaucoma research. If you’re interested in becoming a participant, you can explore glaucoma clinical trials by visiting ClinicalTrials.gov and running a search for “glaucoma.” Be sure to speak with your eye doctor first to determine if joining a clinical trial is a good option for you.
If you have risk factors for glaucoma or have begun to notice symptoms, it is time to schedule a comprehensive eye exam. While you’re there, it’s also a good time to ask any questions you may have, such as:
How often should you, specifically, have eye exams?
What is your IOP (eye pressure) measurement, and what does that mean?
What lifestyle changes do they recommend for your eye health?
If you do have glaucoma, what type is it?
Is it hereditary/are your children at higher risk?
Is it stable or progressing?
Will your current medications prevent certain glaucoma treatments?
Which treatments are you a good candidate for?
What will happen if you opt out of treatment?
Glaucoma. Johns Hopkins Medicine. Accessed March 2023.
Glaucoma. American Optometric Association. Accessed March 2023.
Glaucoma FAQs | Wills Eye Hospital. Wills Eye Hospital. Accessed March 2023.
What is glaucoma? Glaucoma Research Foundation. Accessed March 2023.
Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed March 2023.
Optic nerve. Cleveland Clinic. January 2022.
Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges ahead. International Journal of Molecular Sciences. March 2020.
Eye pressure. EyeSmart. American Academy of Ophthalmology. May 2022.
Intraocular pressure. StatPearls [Internet]. July 2022.
Aqueous humor & vitreous humor. Cleveland Clinic. December 2022.
Glaucoma. National Eye Institute. April 2022.
Types of glaucoma. National Eye Institute. September 2021.
Glaucoma. Cleveland Clinic. November 2022.
Glaucoma warning signs. Glaucoma Research Foundation. May 2022..
Optic nerve cupping. Glaucoma Research Foundation. March 2022.
Glaucoma and the importance of the eye's drainage system. BrightFocus Foundation. April 2018.
The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal Français d'Ophtalmologie. September 2020.
Open angle glaucoma. World Glaucoma Association. Accessed March 2023.
What is chronic angle-closure glaucoma? EyeSmart. American Academy of Ophthalmology. December 2021.
Overview of eye trauma. Merck Manual Professional Version. September 2022.
Drainage system of the eye. World Glaucoma Association. Accessed March 2023.
Ciliary body. A.D.A.M. Medical Encyclopedia [Internet]. Ebix, Inc. September 2021.
Primary open-angle glaucoma. Merck Manual Professional Version. September 2022.
Open angle glaucoma. StatPearls [Internet]. August 2022.
Unconventional aqueous outflow. EyeWiki. American Academy of Ophthalmology. March 2023.
The genetics of glaucoma. Glaucoma Research Foundation. March 2022.
Glaucoma: Facts & figures. BrightFocus Foundation. October 2022.
Angle-closure glaucoma. Merck Manual Professional Version. September 2022.
Plateau iris. EyeWiki. American Academy of Ophthalmology. December 2022.
Primary vs. secondary angle closure glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Normal tension glaucoma. EyeWiki. American Academy of Ophthalmology. April 2022.
Normal tension glaucoma. Wills Eye Hospital. Accessed March 2023.
Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye. February 2018.
Latest developments in normal-pressure glaucoma: Diagnosis, epidemiology, genetics, etiology, causes and mechanisms to management. Asia-Pacific Journal of Ophthalmology. December 2019.
Normal-tension glaucoma. BrightFocus Foundation. August 2021.
Pediatric glaucoma: types, tests and treatments. Optometry Times. May 2020.
Childhood Glaucoma Research Network classification and epidemiology of childhood glaucoma. EyeWiki. American Academy of Ophthalmology. July 2022.
Primary Congenital Glaucoma. EyeWiki. American Academy of Ophthalmology. August 2022.
Buphthalmos. StatPearls [Internet]. December 2022.
Neovascular glaucoma. EyeWiki. American Academy of Ophthalmology. November 2022.
What is neovascular glaucoma? BrightFocus Foundation. July 2021.
Pigment dispersion glaucoma. StatPearls [Internet]. August 2022.
Pseudoexfoliative glaucoma. EyeWiki. American Academy of Ophthalmology. December 2022.
Uveitis. National Eye Institute. November 2021.
Pathogenesis of uveitic glaucoma. Journal of Current Glaucoma Practice. September-December 2018.
Uveitic glaucoma. EyeWiki. American Academy of Ophthalmology. August 2022.
Traumatic glaucoma. StatPearls [Internet]. August 2022.
Traumatic glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Lens induced glaucomas. EyeWiki. American Academy of Ophthalmology. December 2021.
He kept his eye on the ball. Review of Optometry. July 2020.
Angle recession glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Steroid-induced glaucoma. EyeWiki. American Academy of Ophthalmology. March 2022.
Fast facts about vision loss. Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion. December 2022.
Primary open-angle glaucoma. EyeWiki. American Academy of Ophthalmology. December 2021.
What is a glaucoma "suspect"? BrightFocus Foundation. July 2021.
Glaucoma tests. Cleveland Clinic. March 2022.
Tonometry. University of Michigan Health. January 2022.
IOP and tonometry. EyeWiki. American Academy of Ophthalmology. June 2022.
The eye exam for glaucoma. BrightFocus Foundation. July 2021.
Central corneal thickness measurements with different imaging devices: Ultrasound pachymetry, noncontact specular microscopy, and tono-pachymetry. Beyoglu Eye Journal. February 2022.
Diagnostic instruments. In Contact Lens Practice (Third Ed.). 2018.
Diagnosing glaucoma. NYU Langone Health. Accessed March 2023.
Imaging of the optic nerve: What is it and why is it needed? Glaucoma Research Foundation. January 2022.
Glaucoma: diagnosis and management. London: National Institute for Health and Care Excellence (NICE). January 2022.
Ganglion cell complex analysis in glaucoma patients: What can it tell us? Eye Brain. January 2020.
Gonioscopy. Cleveland Clinic. February 2022.
Current situation and progress of drugs for reducing intraocular pressure. Therapeutic Advances in Chronic Disease. December 2022.
Treatments for glaucoma. BrightFocus Foundation. Accessed March 2023.
Omlonti: Uses, dosage, side effects & warnings. Drugs.com. October 2022.
Topical carbonic anhydrase inhibitors. EyeWiki. American Academy of Ophthalmology. March 2023.
Nitric oxide donating anti-glaucoma drugs: advances and prospects. Chinese Journal of Natural Medicines. April 2020.
The role of nitric oxide in the intraocular pressure lowering efficacy of latanoprostene bunod: Review of nonclinical studies. Journal of Ocular Pharmacology and Therapeutics. March 2018.
Effects of selective EP2 receptor agonist, omidenepag, on trabecular meshwork cells, Schlemm’s canal endothelial cells and ciliary muscle contraction. Scientific Reports. August 2021.
Rho kinase inhibitors as a neuroprotective pharmacological intervention for the treatment of glaucoma. Cureus. August 2022.
Neuroprotection in glaucoma. EyeWiki. American Academy of Ophthalmology. April 2020.
Use of rho kinase inhibitors in ophthalmology: A review of the literature. Medical Hypothesis, Discovery & Innovation Ophthalmology Journal. Fall 2018.
Durysta (bimatoprost implant). EyeWiki. American Academy of Ophthalmology. December 2022.
Glaucoma laser and surgery. Wills Eye Hospital. Accessed March 2023.
Does marijuana help treat glaucoma or other eye conditions? EyeSmart. American Academy of Ophthalmology. December 2022.
Treating glaucoma. Glaucoma Research Foundation. Accessed March 2023.
Cannabis is associated with blood pressure reduction in older adults – A 24-hours ambulatory blood pressure monitoring study. European Journal of Internal Medicine. January 2021.
Eye drops vs. laser treatment for glaucoma. BrightFocus Foundation. July 2021.
Minimally invasive glaucoma surgery. StatPearls [Internet]. February 2023.
Glaucoma surgery. National Eye Institute. January 2022.
Glaucoma surgery series: Tube shunt drainage devices. BrightFocus Foundation. July 2021.
Comprehensive adult eye and vision examination, second edition. American Optometric Association. January 2023.
What is glaucoma? Symptoms, causes, diagnosis, treatment. EyeSmart. American Academy of Ophthalmology. December 2023.
Glaucoma caregiving. BrightFocus Foundation. Accessed September 2024.
5 tips for living better with glaucoma. Johns Hopkins Medicine. Accessed September 2024.
New research aims to develop novel therapeutic for glaucoma. Indiana University School of Medicine. January 2024.
With positive initial results, innovative glaucoma therapy moves ahead. BrightFocus Foundation. June 2023.
Page published on Monday, February 25, 2019
Page updated on Tuesday, September 10, 2024
Medically reviewed on Tuesday, April 25, 2023