Secondary glaucoma: Causes, symptoms, types and treatment
Causes of secondary glaucoma
Glaucoma is an eye disease that develops when eye pressure damages the optic nerve. In secondary glaucoma, an existing condition or other outside factor causes eye pressure to go up enough to cause this damage. In primary glaucoma, this happens without any outside factor.
The medical term for eye pressure is intraocular pressure (IOP). Maintaining a certain level of IOP is very important for vision and overall eye health. IOP that is too low or too high can cause serious problems, and everyone’s ideal IOP is slightly different.
Fortunately, the aqueous humor in our eyes usually keeps IOP balanced for us. It does this by flowing into and out of the front part of the eye at a constant, steady rate.
If this steady flow slows down or stops, IOP can rise enough to lead to glaucoma.
In most cases, this happens because aqueous humor can’t drain from the eye quickly enough. It can also happen if aqueous flow is restricted earlier along its pathway or if the eye produces too much aqueous humor.
In primary glaucoma, there is no identifiable cause for these issues. With secondary glaucoma, there are known, specific factors directly affecting aqueous flow and IOP. These factors fall into five broad categories:
Underlying health conditions
Eye infections or inflammation
Previous eye injury
Eye surgery
Medication side effects
Secondary glaucoma can be acute (sudden) or chronic (gradual), depending on its cause. It is much less common than the primary forms. But like primary glaucoma, it very rarely has any early symptoms.
Types of secondary glaucoma
Also like primary glaucoma, the two main types of secondary glaucoma are open angle and angle closure (also called narrow angle). But secondary glaucoma has many more subtypes.
The terms open angle and angle closure describe the basic structural issue within the eye that is disrupting aqueous flow. The names of the subtypes describe the underlying causes of those structural issues. Most of the subtypes can be either open angle or angle closure.
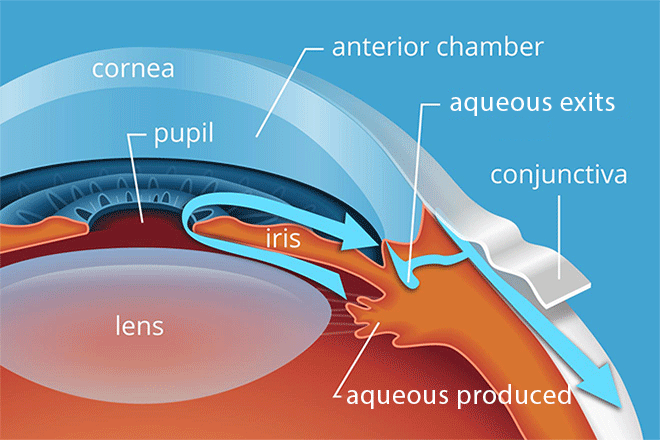
To keep our IOP balanced, the aqueous humor has to be able to flow freely along a specific path through and then out of the eye.
This path begins at the ciliary body, which produces the aqueous. From there, it flows along the underside of the iris, up through the pupil and then out through the drainage angle.
Beyond the angle, it filters through a tissue called the trabecular meshwork and other drainage structures. Then it is eventually reabsorbed into the body.
A smaller amount of fluid also leaves the eye through uveoscleral outflow. This fluid essentially seeps into and through the eye wall.
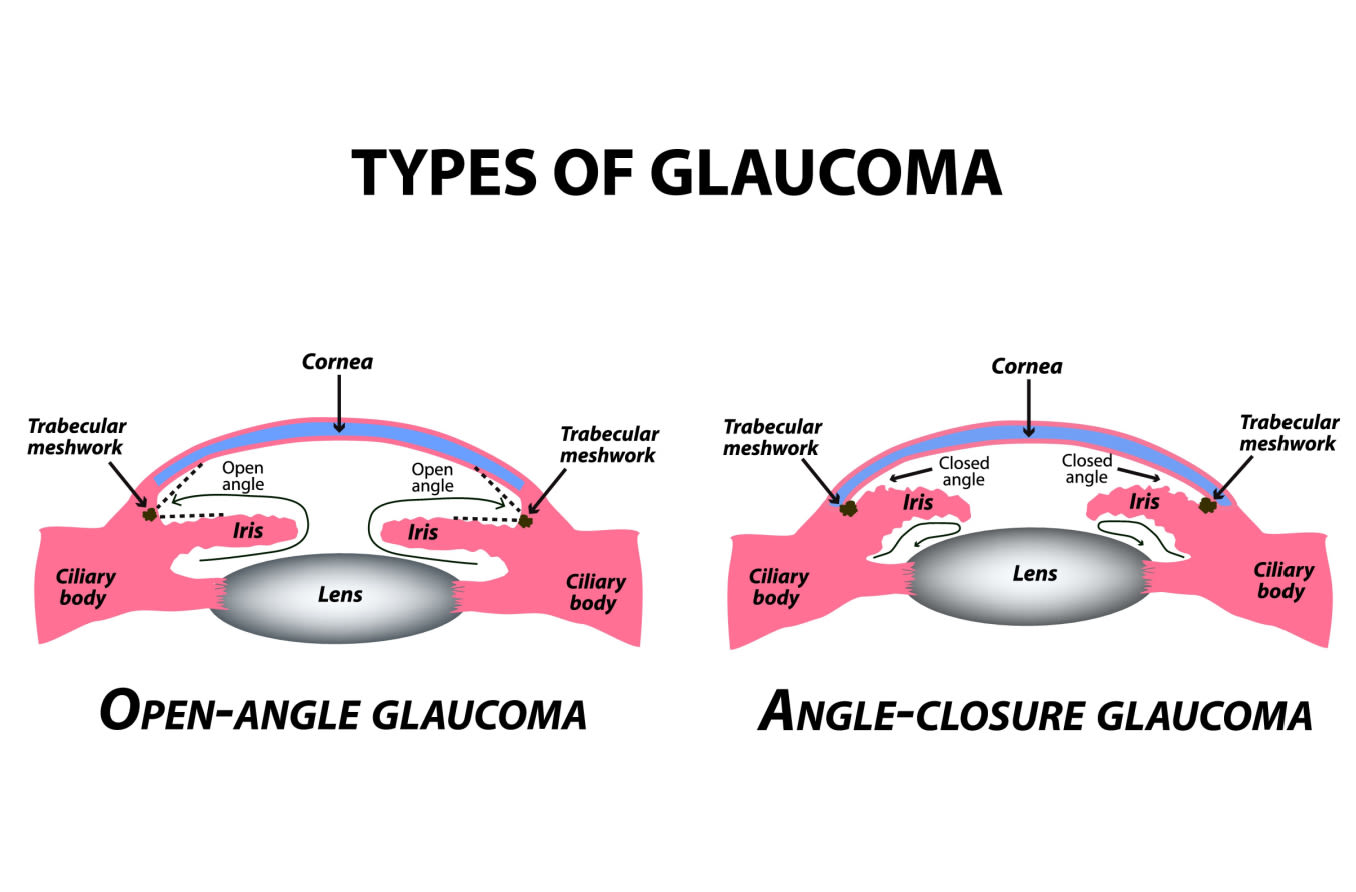
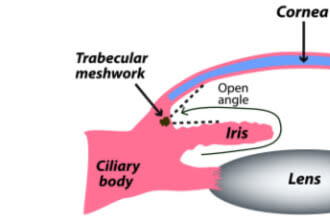
In open-angle forms, the aqueous flow disruption usually occurs in the trabecular meshwork.
In angle-closure forms, the aqueous can’t leave the eye because the iris is blocking the drainage angle.
LEARN MORE about glaucoma causes
Secondary open-angle glaucoma
The drainage angle is a delta-shaped notch of space that surrounds the outside edge of the iris. It is formed by the iris on one side and the edge of the cornea on the other. This angle helps to funnel aqueous fluid into the trabecular meshwork.
When glaucoma develops in eyes that don’t have any obstruction in this notch of space, it is called open-angle. In most of these cases, outflow resistance in the trabecular meshwork causes IOP to increase.
The primary open-angle forms happen without any known cause. But in secondary open-angle glaucoma, something directly causes this resistance. Many conditions and outside factors can damage the trabecular meshwork or cause it to become clogged or inflamed.
Secondary angle-closure glaucoma
Angle closure occurs when the iris encroaches on or blocks the drainage angle. A narrow or closed angle will significantly slow down aqueous flow and cause IOP to spike.
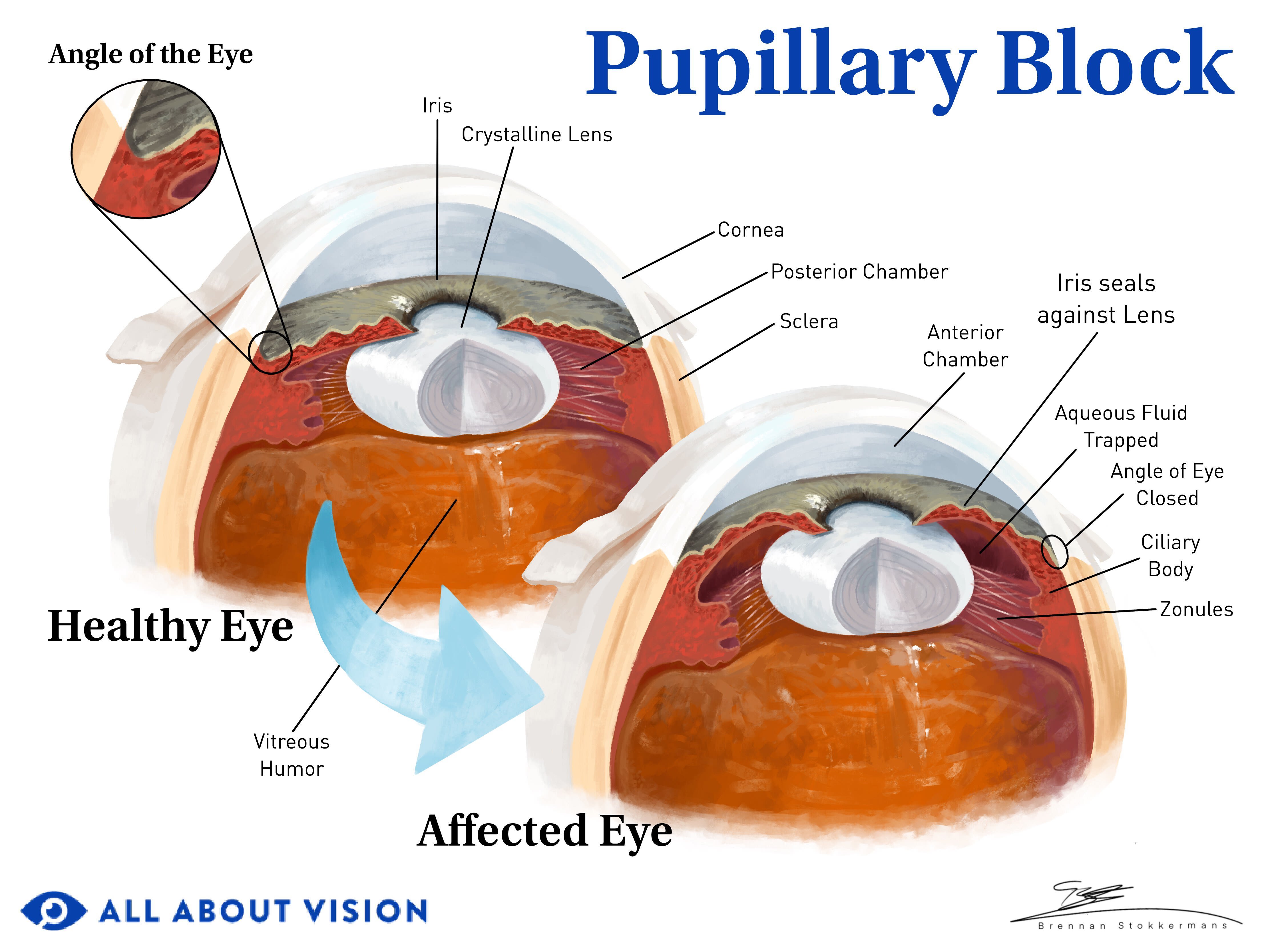
Click on illustration to enlarge.
Often, pressure or crowding from behind the iris pushes it into the angle space. An enlarged or displaced lens can trap aqueous behind the iris if there isn’t enough space for it to flow through the pupil.
The pressure of the trapped aqueous causes the iris to bow forward and close the angle. This is called pupillary block.
While most secondary subtypes can be open- or closed-angle, secondary closed-angle glaucoma is less common. The subtypes that can be either/or usually begin with open angles and only progress to angle closure due to severe or chronic inflammation.
The main exceptions to this are closed angles caused by eye injuries and eye infections. A blow to the eye, even one that seems relatively minor, can dislocate the lens and quickly cause angle closure. Eye infections can also cause acute angle closure if the ciliary body and/or the iris are inflamed and swollen.
Pseudoexfoliative glaucoma (also called exfoliative glaucoma)
Usually secondary open-angle but can progress to secondary angle-closure glaucoma
Chronic and acute
This form develops as a result of an age-related condition called pseudoexfoliation syndrome (PEX). In PEX, the body sheds protein molecules, producing a flakey, dandruff-like material. These flakes accumulate in organs throughout the body, including in the eyes.
Eventually, the eyes' drainage structures can become clogged or blocked by these flakes. When this happens, IOP increases, which can lead to pseudoexfoliative glaucoma (PXG).
Buildup of these flakes can also affect the iris and lens, limiting their ability to function. PEX can weaken the tiny fibers that hold the lens in place. As these fibers become looser, the lens can move forward and crowd the iris into the drainage angle. In these cases, PXG is a type of secondary angle-closure glaucoma.
PXG is the most common type of secondary glaucoma. However, it seems to affect some populations more than others. People with Scandinavian, Mediterranean, Arabic or Japanese backgrounds tend to have higher rates of both PEX and PXG.
Neovascular glaucoma
Usually open-angle but can progress to secondary angle-closure glaucoma
Chronic
Certain conditions, such as diabetes, can significantly affect the blood supply to the eyes. The body may then try to correct this issue by growing new blood vessels. This is called neovascularization, or proliferative disease.
In people who have diabetes, this process is called proliferative diabetic retinopathy. Poor blood flow to the retina causes an overgrowth of new, but unhealthy, blood vessels on the retina and iris. Because the new blood vessels are weak and unhealthy, they also break and leak easily.
If left untreated, the overgrowth and leakage eventually clog the drainage pathway and cause IOP to increase.
Neovascularization of the iris can also cause an abnormal membrane to form in the space between the iris and trabecular meshwork. This membrane then pulls and binds the two together, closing off parts of the drainage angle. This is called neovascularization of the angle. These closed-off areas (called synechiae) can lead to secondary angle-closure glaucoma.
ICE syndrome-associated glaucoma
Usually secondary closed-angle glaucoma but can develop with an open angle
Chronic
Usually unilateral (only affects one eye)
Iridocorneal endothelial (ICE) syndrome is a rare corneal tissue disorder. It can cause severe corneal swelling, iris deformation and secondary glaucoma. Though ICE itself is quite rare, this syndrome results in glaucoma in about 50% of cases.
In people who have ICE syndrome, abnormal cells from the cornea multiply and spread to other areas, including the drainage angle and iris.
As these cells move into the drainage angle, less and less fluid can pass through. And, since these cells are leaving the cornea, the cornea becomes both thinner and swollen, which can also increase IOP.
Sometimes, these two issues alone can damage the optic nerve. In these cases, glaucoma develops while the angle is still technically open. But often, ICE syndrome eventually leads to angle closure. This happens in a process that is very similar to angle closure in neovascular glaucoma.
The abnormal corneal cells form a continuous membrane as they spread through the eye. It connects the cornea, drainage angle, trabecular meshwork and iris. When it contracts, it pulls and stretches the iris toward the meshwork and binds them together in areas called synechiae. These synechiae close off the angle.
The swelling and stretching can also cause significant damage to the cornea and iris. There are three subtypes of ICE based on the degrees of this damage, but each subtype can lead to glaucoma.
Pigmentary glaucoma
Secondary open-angle glaucoma
Chronic
This type of secondary glaucoma is caused by a condition called pigment dispersion syndrome (PDS). In PDS, tiny fragments of pigment from the iris come free and wash into the eye's drainage angle.
This tends to happen due to the relative placement in the eye of the iris and lens. If they are too close together, they can rub against each other. Certain movements, head postures and even accommodation can cause contact. The friction during this contact is what causes the iris pigment to come free.
Pigmentary glaucoma is relatively rare. It most commonly affects people who have myopia (nearsightedness) — usually white males. Myopic eyes tend to be longer than average from front to back. In some cases, this extra length may allow the iris to bow backward too far and touch the lens.
Traumatic glaucoma
Secondary open-angle, angle recession or secondary angle-closure glaucoma
Chronic and acute
This form can develop when trauma to the eye, such as an injury or surgery, causes increased IOP. Both blunt-force trauma (like being hit in the eye) and penetrating trauma (when a foreign object punctures the eye) can lead to traumatic glaucoma (TG).
TG can be either open- or closed-angle, depending on which tissues and structures are affected. For example, the injury could displace the lens and/or iris and disrupt fluid movement. This could lead to secondary angle-closure glaucoma. An injury can also damage the eye’s drainage structures or cause inflammation that impairs them.
Another common type of TG is called angle-recession glaucoma. The force of blunt trauma can drive the drainage angle backward, tearing tissues in the ciliary body and drainage structures. As the torn tissues heal and form scars, less and less aqueous can flow through.
Penetrating eye trauma can also trigger an autoimmune reaction in some people. When this happens, inflammatory cells can clog the eye’s drainage structures and lead to increased IOP. In the rare cases of secondary glaucoma following eye surgery, this immune response tends to be the cause.
It’s important to note that TG can have an early onset or a late onset. Some injuries can cause IOP to increase right away. But it is very common for TG to develop 10 or more years after an injury.
For this reason, it’s very important for anyone who has had an eye injury to get yearly comprehensive eye exams. TG can develop well after an eye injury is healed and forgotten — even those that seem trivial.
Uveitic glaucoma
Secondary open-angle or secondary angle-closure glaucoma
Chronic and acute
The uvea is a continuous tract of tissue that makes up three very important parts of the eye: the iris, ciliary body and choroid. When any of these parts become inflamed or infected, it’s called uveitis.
Uveitis can be divided into four types, depending on the area(s) affected: anterior, intermediate, posterior and diffuse. The most common type, anterior uveitis, involves the iris and ciliary body. This is the type most commonly associated with uveitic glaucoma.
However, all types of uveitis can disrupt aqueous flow in one or more ways and lead to glaucoma. The open-angle forms are typically due to inflammatory cells and their debris released into the front of the eye. They can clog and even permanently damage the eye’s drainage structures.
Uveitis can also lead to secondary angle-closure glaucoma. The severe inflammation can trigger neovascularization and/or the formation of synechiae. The synechiae may form in the angle, closing it off, or between the iris and lens, leading to pupillary block.
The most common treatment for uveitis is corticosteroid eye drops, but steroids are known to worsen glaucoma. This, combined with the complex ways uveitis can increase IOP, makes uveitic glaucoma one of the most difficult to treat.
Posner-Schlossman syndrome (PSS) uveitic glaucoma
Secondary open-angle glaucoma
Chronic
Unilateral (only affects one eye)
PSS is a relatively rare condition in which a person has recurring attacks of anterior uveitis. IOP spikes during these acute episodes, and, over time, this can lead to secondary glaucoma.
Also called glaucomatocyclitic crisis, PSS uveitis is different from other types of uveitis in a few important ways:
1 - It’s recurrent and unpredictable. Recurrent uveitis is typically related to an autoimmune issue, but this is not the case with PSS. There are no known underlying causes or flare-up triggers for PSS.
Each repeated episode of acute uveitis may last less than a day, or it may last for months. They may recur every few weeks or months, or there may be years between them.
2 - Its symptoms are milder. Acute anterior uveitis usually causes significant eye pain, redness, light sensitivity and blurred vision. With PSS, inflammation and its typical symptoms are minimal.
Most patients only notice minor eye discomfort and/or blurry vision. Many don’t have symptoms at all.
3 - It raises IOP drastically. All types of uveitis can have a dramatic impact on IOP. Usually, the increase in eye pressure it causes is directly related to the degree of inflammation in the eye.
But with PSS, eye pressure during an attack can be double or even quadruple the normal range, even without inflammation. In most cases, IOP does go back to normal between attacks. However, the spikes in eye pressure can cause permanent optic nerve damage.
There is not much research to show how rare PSS truly is or how often it causes glaucoma. But the studies that do exist estimate that PSS affects around two people per 100,000, and about a quarter of those people develop glaucoma.
Steroid-induced glaucoma
Secondary open-angle glaucoma
Chronic and acute
This secondary form can develop when IOP increases as a response to corticosteroids. In some people, steroids can cause physical changes within the eyes’ drainage structures and increase abnormal cell debris. These problems raise IOP and can lead to glaucoma.
People whose eyes are affected this way are called steroid responders. Experts estimate that 30%-40% of people are high or moderate responders. Steroid responders are at higher risk, but anyone can develop steroid-induced glaucoma. This is because even a low responsiveness may increase IOP enough to damage the optic nerve.
Other risk factors for steroid-induced glaucoma include:
A family history of glaucoma
Diabetes
Connective tissue diseases
Keratoplasty (corneal transplant)
Age (6 years old or younger)
All types of steroids can have this effect, whether they are prescription or OTC. Steroid eye drops and eye ointments tend to cause the strongest responses. However, pills, nasal sprays and injections can also raise IOP.
Topiramate-induced glaucoma
Secondary angle-closure glaucoma
Acute
Another medication that can increase IOP is topiramate. Doctors prescribe topiramate to treat seizure disorders and to prevent migraine headaches. It is also used to treat neuropathic pain and several mental and behavioral disorders.
A possible side effect of this drug is swelling of the choroid, the middle layer of the eye wall. This swelling can squeeze the back of the eye, forcing the lens and iris forward. In very rare cases, this can cause secondary angle-closure glaucoma.
The forward movement of the lens also causes acute myopia (sudden nearsightedness). If you notice a blurring of your vision after starting topiramate, see an eye doctor immediately.
Childhood secondary glaucoma
Secondary open-angle or secondary angle-closure glaucoma
Chronic and acute
Secondary glaucoma can develop in children for many of the same reasons it develops in adults. For example, eye injuries, infections and steroid eye drops can all raise IOP in children and potentially lead to optic nerve damage.
The most common causes of secondary childhood glaucoma vary among different populations. But the top two causes tend to be eye injury and eye surgery. The most common type to develop due to eye surgery is called aphakic glaucoma. It can develop in children with pediatric cataracts after they have cataract surgery.
Retinopathy of prematurity is another eye disease that increases a child’s risk. With this condition, glaucoma can develop as a result of the retinopathy itself or as a complication after surgery to treat it.
Childhood glaucoma can also develop secondarily to genetic conditions, including:
Sturge-Weber syndrome
Many other genetic conditions are also associated with glaucoma.
Signs and symptoms
Glaucoma almost never has any signs or symptoms until well into its progression. It can take many years before vision loss is noticeable, and it cannot be reversed.
The first vision changes someone may notice are that they need more light to read or that colors seem duller. Eventually, they may notice that their peripheral vision is shrinking.
The damage to the optic nerve usually progresses very slowly. It is also completely painless because the retina and optic nerve don’t have pain receptors. And, as the damage gradually erodes vision, our brains are able to “fill in the gaps” for a while.
Most people won’t notice any changes to their vision until there is too much optic nerve damage for the brain to make up for.
However, there are a few exceptions among the secondary forms of the condition. Chronic and acute angle closure can both cause symptoms. So, any type of secondary angle-closure glaucoma can potentially have symptoms.
Acute angle closure happens suddenly and causes severe eye pain, headaches, blurry vision and eye redness. The pain is due to the rapid, drastic increase in pressure in the front of the eye during an acute attack.
It may also cause vomiting and seeing multi-colored halos around lights. Acute angle closure can cause blindness within hours and requires immediate treatment.
These symptoms are also possible in chronic and intermittent angle closure. In secondary glaucomas that begin with open angles but progress to angle closure, mild symptoms may come and go until the angle is fully blocked.
Diagnosis and treatment
Only an optometrist or ophthalmologist can detect and diagnose glaucoma. They do this with comprehensive eye exams and other more specialized glaucoma tests.
High IOP and optic nerve damage very rarely cause any symptoms or outward, visible signs. But there are signs inside the eye that experienced eye doctors can see during these exams.
This is one reason it's so important to have yearly eye exams. They give you and your eye doctor the advantage of much earlier detection and treatment. People at higher risk for primary or secondary glaucoma may need to have eye exams more often.
If treatment begins early enough, it can be possible to avoid noticeable vision loss.
The treatments for primary and secondary glaucoma are largely the same. This is because the goal of all glaucoma treatment is to lower intraocular pressure. The main difference in treating a secondary form is managing its underlying cause.
In secondary glaucoma, the elevated IOP and the condition causing it should both be addressed. For example, diabetes and high blood pressure must be under control for glaucoma treatment to be successful.
The best treatment plan for each person depends on the type of glaucoma they have. Other health conditions, previous eye surgery and target IOP are also factors.
For many patients, prescription eye drops and/or laser procedures work well to control IOP. More advanced cases often require microsurgery.
Common laser procedures and surgeries include:
Trabeculoplasty – This is the most common laser procedure for treating open-angle glaucoma (OAG). The specialist uses targeted laser energy to create better drainage openings in the trabecular meshwork.
Laser peripheral iridotomy – This is the most common laser procedure used for narrow or closed angles. The specialist uses a laser to make a very small hole in the iris. This releases aqueous trapped behind the iris and relieves the pupillary block.
MIGS – MIGS stands for minimally invasive glaucoma surgery. They are a common choice for mild-to-moderate cases of OAG that don’t respond to drugs or laser treatment. MIGS procedures use stents, shunts and other methods to allow the aqueous to bypass the trabecular meshwork.
Patients with advanced glaucoma or who don’t respond to other treatment will need conventional surgery. These procedures are also considered microsurgery. However, they are slightly more invasive than laser and MIGS.
Common conventional surgeries include:
Trabeculectomy – This procedure is used to treat OAG. The surgeon makes an incision through the conjunctiva and sclera to remove a tiny piece of the trabecular meshwork. Then they reposition a flap of conjunctival tissue over the incision. Aqueous can now drain through this new path. The flap collects fluid and forms a bleb to regulate the amount of outflow.
Peripheral iridectomy – This procedure is used to treat angle-closure glaucoma. The surgeon removes a tiny piece of tissue from the outer edge of the iris. As with laser iridotomy, this releases fluid behind the iris to relieve pupillary block.
LEARN MORE about glaucoma medications and glaucoma surgery
Can secondary glaucoma be cured?
Unfortunately, glaucoma cannot be cured or reversed. The optic nerve is a part of our central nervous system, which means it can't regenerate or replace its cells. No matter what type of glaucoma a person has, the resulting vision loss can’t be undone.
However, it may be possible — in some cases — to cure or correct the condition causing secondary glaucoma. This can return IOP to a healthy level and stop any further vision loss.
Can it be prevented?
There is no way to prevent primary glaucoma, but some types of secondary glaucoma may be avoidable.
The best way to prevent secondary glaucoma is to manage its underlying cause. When diseases like diabetes and hypertension are under control, there is less risk for the complications that raise IOP.
Traumatic glaucoma is preventable with basic safety measures. Safety glasses and helmets can help protect you from eye or head injuries that can lead to glaucoma.
Seeking medical care right away for eye infections and inflammation, especially uveitis, can prevent a spike in IOP. Any time you have eye pain or extreme eye redness, see an eye doctor as soon as possible.
It's also very important to avoid overuse of corticosteroids. Even over-the-counter forms of this drug can raise IOP. Always tell your eye doctor about any glaucoma in your family history and any prescription or OTC steroids you use.
Unfortunately, there are still many types of secondary glaucoma that cannot be prevented. But it is possible to prevent the vision loss they cause or stop vision loss from progressing.
The most effective way to avoid losing sight to glaucoma is early detection and treatment.
Everyone 6 years of age and older should have yearly comprehensive eye exams. However, people who have known risk factors for glaucoma may need more frequent exams.
Depending on a person's level of risk, they may need exams every six months or even more often.
READ NEXT: 8 Things Your Eyes Can Reveal About Your Health
Glaucoma. American Optometric Association. Accessed February 2023.
What is glaucoma? Glaucoma Research Foundation. Accessed April 2023.
What is glaucoma? In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Secondary glaucoma. In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Eye pressure. EyeSmart. American Academy of Ophthalmology. May 2022.
Intraocular pressure. StatPearls [Internet]. July 2022.
Glaucoma and the importance of the eye's drainage system. BrightFocus Foundation. April 2018.
The trabecular meshwork: structure, function and clinical implications. A review of the literature. Journal Français d'Ophtalmologie. September 2020.
Secondary glaucomas. Glaucoma UK. June 2020.
Open angle glaucoma. World Glaucoma Association. Accessed April 2023.
Unconventional aqueous outflow. EyeWiki. American Academy of Ophthalmology. March 2023.
Low-tension glaucoma: an oxymoron in ophthalmology. Preventing Chronic Disease. January 2019.
Angle-closure glaucoma. Merck Manual Professional Version. September 2022.
Primary vs. secondary angle closure glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Traumatic glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Ectopia lentis. Cleveland Clinic. December 2022.
Pseudoexfoliative glaucoma. EyeWiki. American Academy of Ophthalmology. December 2022.
Pseudoexfoliation syndrome and glaucoma. StatPearls [Internet]. February 2023.
Neovascular glaucoma. EyeWiki. American Academy of Ophthalmology. November 2022.
What is neovascular glaucoma? BrightFocus Foundation. July 2021.
Synechiae. EyeWiki. American Academy of Ophthalmology. September 2022.
Iridocorneal endothelial syndrome and secondary glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
What is iridocorneal endothelial syndrome (ICE)? EyeSmart. American Academy of Ophthalmology. November 2022.
The iridocorneal endothelial syndrome. Survey of Ophthalmology. September 2018.
What is irido corneal endothelial syndrome (ICE)? Glaucoma Research Foundation. August 2022.
Glaucoma and ICE syndrome. BrightFocus Foundation. August 2021.
Pigment dispersion glaucoma. StatPearls [Internet]. August 2022.
Traumatic glaucoma. StatPearls [Internet]. August 2022.
Angle recession glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
Lens induced glaucomas. EyeWiki. American Academy of Ophthalmology. December 2021.
He kept his eye on the ball. Review of Optometry. July 2020.
Uveitis. National Eye Institute. November 2021.
Glaucomato-cyclitic crisis. ICD-11 for Mortality and Morbidity Statistics. World Health Organization. January 2023.
Glaucomatocyclitic crisis (Posner-Schlossman syndrome). EyeWiki. American Academy of Ophthalmology. May 2023.
Posner Schlossman syndrome. StatPearls [Internet]. February 2023.
Acute anterior uveitis. EyeWiki. American Academy of Ophthalmology. December 2022.
Glaucomatocyclitic crisis. Kahook's Essentials of Glaucoma Therapy. April 2021.
Anterior uveitis. American Optometric Association. Accessed May 2023.
Posner-Schlossman syndrome. Cureus. January 2020.
Uveitic glaucoma. EyeWiki. American Academy of Ophthalmology. August 2022.
How to manage uveitic glaucoma. Review of Ophthalmology. April 2019.
Uveitis and glaucoma: Is there a connection? BrightFocus Foundation. August 2021.
Host-derived cytotoxic agents in chronic inflammation and disease progression. International Journal of Molecular Sciences. February 2023.
Pathogenesis of uveitic glaucoma. Journal of Current Glaucoma Practice. September-December 2018.
Steroid-induced glaucoma. EyeWiki. American Academy of Ophthalmology. March 2022.
Steroid induced glaucoma. StatPearls [Internet]. February 2023.
Canaloplasty in corticosteroid-induced glaucoma. Preliminary results. Journal of Clinical Medicine. February 2018.
Topiramate. StatPearls [Internet]. January 2023.
The ophthalmic side effects of topiramate: A review. Cureus. August 2022.
Topiramate-induced angle closure. EyeRounds.org. April 2019.
Pediatric glaucoma following cataract surgery. EyeWiki. American Academy of Ophthalmology. April 2023.
Case report: Glaucoma in an infant with retinopathy of prematurity. Frontiers in Pediatrics. December 2021.
Childhood glaucoma: Diagnosis, treatment, and hope. Glaucoma Research Foundation. May 2022.
Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness – a review. Restorative Neurology and Neuroscience. November 2018.
Is glaucoma painful? BrightFocus Foundation. July 2021.
Drug-induced acute angle-closure glaucoma: Raising your index of suspicion. Biomedical Journal of Scientific & Technical Research. June 2021.
Comprehensive adult eye and vision examination, second edition. American Optometric Association. January 2023.
Treatments for glaucoma. BrightFocus Foundation. Accessed April 2023.
Laser peripheral iridotomy. EyeWiki. American Academy of Ophthalmology. December 2022.
Lifestyle habits and glaucoma. EyeWiki. American Academy of Ophthalmology. January 2022.
Minimally invasive glaucoma surgery. StatPearls [Internet]. February 2023.
Surgical iridectomy. Kahook's Essentials of Glaucoma Therapy. October 2019.
Optic nerve regeneration. BrightFocus Foundation. August 2021.
The quest to restore lost vision and cure glaucoma. Glaucoma Research Foundation. May 2022.
Steroids and glaucoma: What’s the connection? Glaucoma Research Foundation. March 2022.
What is a glaucoma "suspect"? BrightFocus Foundation. July 2021.
Page published on Friday, June 12, 2020
Page updated on Tuesday, August 8, 2023
Medically reviewed on Saturday, May 13, 2023