Glaucoma causes: What we know about intraocular pressure (IOP) and optic nerve damage
Understanding glaucoma and eye anatomy
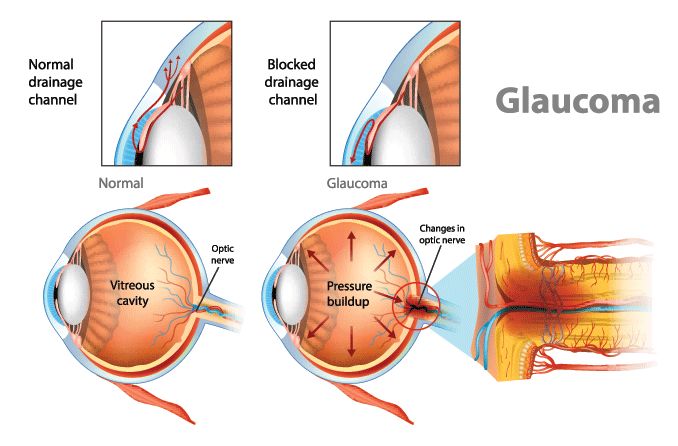
Glaucoma is a progressive eye disease that leads to vision loss and, often, blindness. It develops when internal eye pressure is too high for the optic nerve to withstand. In most cases, there aren’t any known causes of glaucoma. But it is usually related to problems in the eye’s drainage pathway.
If aqueous humor can’t flow out of the eye properly, it causes intraocular pressure (IOP) to rise. Higher IOP can eventually cause serious damage to the optic nerve — the nerve that carries information from the eyes to the brain.
Experts know that problems in the drainage pathway raise IOP, and they know that higher IOP can harm the optic nerve. But in most cases, they don’t know why the drainage problems develop. And they don’t know exactly why IOP affects the optic nerve. This is why eye doctors talk about risk factors rather than causes of glaucoma.
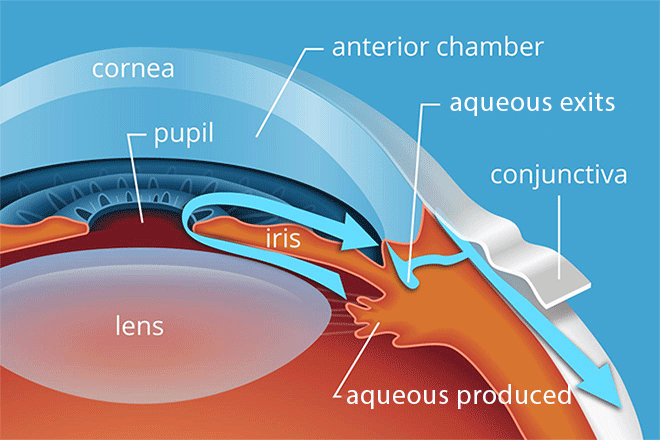
The optic nerve is located at the very back of the eye. But the structures in the eye’s anatomy that produce and regulate the aqueous fluid are located toward the front. This front area of the eye is called the anterior segment.
The anterior segment includes all of the eye’s structures and organs from the cornea to the lens. It is divided into two parts, which are called the anterior chamber and the posterior chamber:
Anterior chamber –
This is the space between the inside surface of the cornea and the front surface of the iris.
It includes the drainage angle, which is located where the outer edge of the iris meets the cornea.
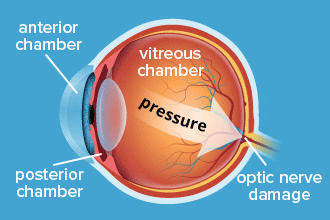
Posterior chamber –
This is the space between the back surface of the iris and the front surface of the lens.
It includes the ciliary body, which produces the aqueous fluid and adjusts the lens for focusing.
The ciliary body continually produces new aqueous humor — at about one-millionth of a liter per minute. This fluid provides vital nutrients to the lens and cornea and helps to keep the anterior segment clean.
Since there is always new fluid entering the anterior chamber, a clear drainage pathway is critical for maintaining a healthy IOP. Some of our aqueous seeps out through the tissues of the eye wall. This is the uveoscleral pathway. But the primary pathway is through the drainage angle:
When aqueous humor leaves the ciliary body, it needs to flow over the surface of the lens and out through the pupil into the anterior chamber.
From there, it needs to be able to leave the eye through the drainage angle.
Beyond the angle, the aqueous humor should flow out through a tissue called the trabecular meshwork and then into Schlemm’s canal.
Typically, drainage problems occur in either the trabecular meshwork (TM) or in the drainage angle. The TM is a complex, sponge-like structure that acts as a sort of filter. Sometimes, this tissue can become clogged, stiff or damaged in some way.
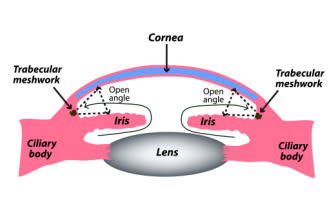
When glaucoma develops even though there are no visible obstructions in the angle, it’s called open-angle glaucoma. In most cases, this form is due to resistance in the TM on a microscopic level.
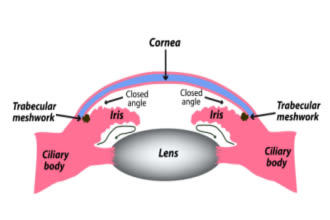
When glaucoma develops because the angle is obstructed, it’s called narrow-angle or angle-closure glaucoma. In this form, the angle is blocked or partially blocked by the outer edge of the iris.
Both of these problems affect how fluid flows through the front of the eye. But the increase in intraocular pressure (IOP) affects the whole eye, including the optic nerve at the back.
The optic nerve is a bundle of more than 1 million axons, or fibers, that carries visual input to the brain from cells in the eye. These cells are part of the retina and are called retinal ganglion cells (RGCs).
RGC fibers all come together at the very back of the eye in an area called the optic disc. This is where they form into a bundle (the optic nerve) and leave the eye.
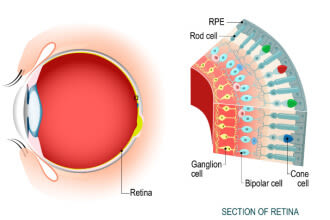
When intraocular pressure is too high for too long, RGCs begin to die.
These RGCs can no longer send visual input to the brain, which results in vision loss.
RGCs that die are never replaced with new cells. So, unfortunately, glaucoma causes irreversible vision loss.
Optic nerve damage can occur with many conditions besides glaucoma. However, the type of damage that glaucoma causes has some distinctive signs that eye doctors can see when they examine the eye. They use these signs, along with other tests and the patient’s medical history, to diagnose glaucoma.
The most common forms develop when IOP rises due to problems somewhere along the aqueous humor’s pathway. The type of glaucoma a person is diagnosed with usually depends on where and how this pathway is affected.
However, it isn’t always related to high or increased eye pressure. In some people, eye pressure in the normal range can still lead to the death of RGCs. This is called normal-tension glaucoma (NTG). It’s also possible to have above-average or high IOP but never develop glaucoma.
How do you get glaucoma?
Glaucoma is not a contagious disease; you can’t get it from being near someone who has it. And there are no specific behaviors or environmental influences that are known to be causes of glaucoma. Even so, it affects close to 2% of the U.S. population over age 40, and anyone can develop the condition.
There are several different types of glaucoma, but they can be divided into two broad categories. The first category is primary glaucomas. They are called primary because they develop on their own, without any known cause. Most cases fall into this category.
The other category is secondary glaucomas. They are called secondary because they develop as a result of something else that came first. Causes of secondary glaucoma range from congenital birth defects to eye infections.
Having high internal eye pressure (called ocular hypertension) is one major risk factor in both categories. In the majority of cases, it is clear that high eye pressure ultimately led to cell death in the optic nerve.
However, for many people, moderately high IOP never affects these cells. Others develop glaucoma even though their IOP has always been within a normal range. And in some patients, the nerve cell damage keeps progressing after treatment lowers their IOP.
Even with these differences, elevated IOP is still the most important risk factor for glaucoma. The higher the IOP, the higher the risk — and very small increases in IOP can raise a person’s risk by up to 10%.
Researchers have not yet been able to solve why eye pressure affects the optic nerve differently in different people. Some of the most long-standing theories are that eye size, eye wall thickness and the optic nerve’s blood supply may be involved. Another is that some optic nerves may simply be more sensitive.
Relatively newer research over the last 20 years points to other possible causes of glaucoma at the molecular level.
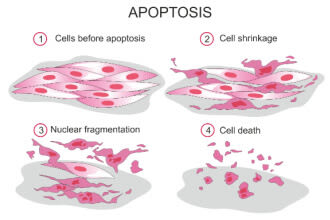
When retinal ganglion cells die, it often happens through a process called apoptosis. This is a natural process that causes aging, damaged or abnormal cells to self-destruct. In glaucoma, stress on the optic nerve cells from IOP triggers this process.
There is evidence that differing amounts of stress on these cells can cause molecular changes in their extracellular matrix (ECM).
The ECM surrounds cells and is made up of proteins that support cell structure and regulate cell behavior.
Researchers theorize that various changes in the ECM could be responsible for if and when apoptosis is triggered.
There is also evidence that one of the causes of glaucoma may be an autoimmune dysfunction.
Researchers have found that retinal ganglion cells produce heat shock proteins (HSPs) in response to stress.
HSPs typically act as protection for cells. But in glaucoma, it appears that these HSPs are triggering an autoimmune T-cell response.
The flood of T-cells may be what ultimately leads to RGC death.
Though there are no definitive answers about the causes of glaucoma, lowering IOP is the main target in treatments. Most, if not all, forms of the disease are related to IOP in some way.
Primary open-angle glaucoma (POAG)
This form usually develops due to structural changes in the trabecular meshwork (TM) or, less often, in Schlemm’s canal. It’s called open-angle because nothing is blocking or restricting the eye’s drainage angle.
Instead, the tissues of the TM become stiff, or the number of its sponge-like pores decreases. These changes make it difficult for aqueous humor to pass through, and then IOP goes up.
Primary open-angle glaucoma (POAG) is the most prevalent type in the U.S., accounting for up to 95% of all glaucoma. It is the second-highest cause of blindness among Black Americans after cataracts. For white Americans, it is the third-highest cause of blindness after AMD and cataracts.
If POAG is detected early, it’s possible for many patients to avoid significant vision loss. But POAG rarely has symptoms, and it progresses very slowly. An estimated 50% of people never realize they have it until they experience significant loss of vision. The only way to catch it early is to have yearly comprehensive eye exams.
Primary angle-closure glaucoma (PACG)
This form is also called narrow-angle glaucoma. It develops when the outer edge of the iris shifts and blocks the drainage angle. Though it gets its name from this blockage, it usually results from a problem earlier in the aqueous outflow path.
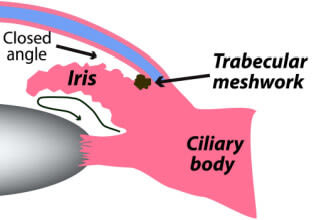
The underlying cause of narrow angles is usually pressure behind the iris that causes the iris to bow forward. When the iris is bowed, its outer edge is pushed forward and toward the angle. And its inner edge, which forms the pupil, is pulled backward toward the lens.
With the pupil’s edge closer to the lens, less fluid can pass through its opening. This is called pupillary block. Pupillary block causes fluid to accumulate behind the iris and leads to even more bowing and angle narrowing.
In most cases, eyes that develop pupillary block and PACG already have less space between the lens and iris.
It may be due to anatomical factors, such as having naturally large lenses or thicker irises. Having smaller-than-average eyes can have the same effect if the lens and iris are too crowded in the small space. Age plays a role as well — the lens of the eye continues to grow as we get older.
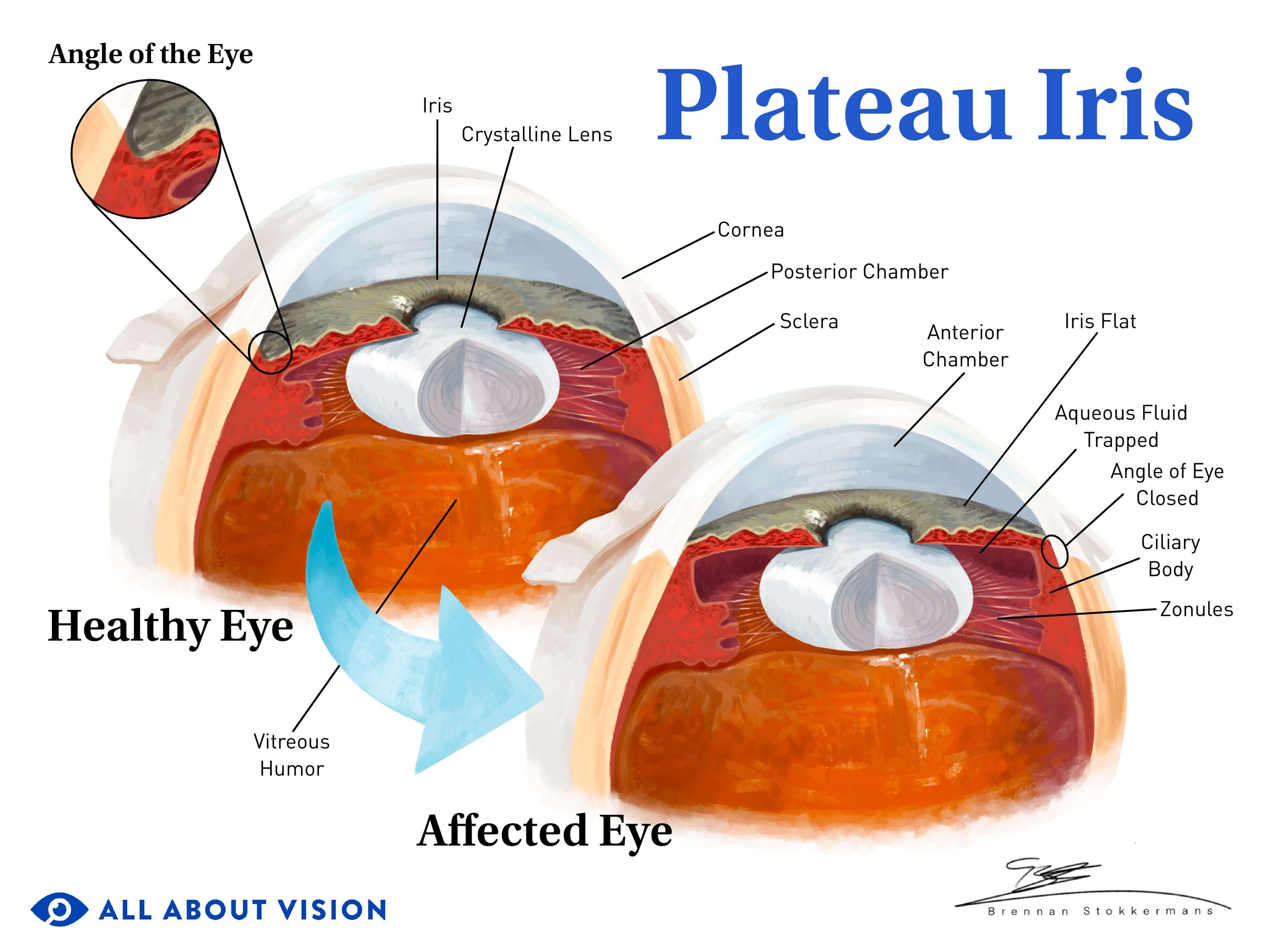
PACG can also occur without pupillary block. Some eyes have naturally narrow angles due to an anatomical trait known as plateau iris.
In these eyes, there is plenty of space between the lens and iris. Instead, the size or forward placement of the iris and/or ciliary body crowd the drainage angle.
The most common treatment for angle-closure glaucoma — a peripheral iridotomy — does not work for plateau iris. It is difficult to diagnose and requires an eye doctor who is experienced in gonioscopy. Misdiagnosis can lead to rapid vision loss.
ACG is usually chronic, meaning it progresses slowly, but it can also be acute. The chronic form is the second-most common after POAG and rarely has symptoms.
The acute (sudden) form is much less common, but it does have symptoms and is very serious. An acute attack can cause intense eye pain, blurry vision, and nausea or vomiting. Untreated acute angle-closure glaucoma causes blindness within hours or days. It is a medical emergency that needs to be treated immediately.
Primary congenital glaucoma (PCG)
This form occurs due to problems during the development of the eye’s drainage system. Babies with primary congenital glaucoma (PCG) often have obvious signs of the condition at birth, such as abnormally large eyes or clouded corneas.
This is because the built-up fluid and pressure has stretched and enlarged the eyes. But some babies born with PCG have later onsets of these signs that range from a few weeks up to around two years.
PCG is one of two types of primary childhood glaucoma. The other is juvenile open-angle glaucoma (JOAG). It is also congenital, but its onset tends to be later in early childhood. Children diagnosed with JOAG are less likely to have enlarged eyes due to this later onset.
Low-tension or normal-tension glaucoma (NTG)
When optic nerve damage develops in eyes that have open angles and typical IOP, it is called normal-tension glaucoma. It is often categorized as a type of POAG.
However, because of its unknown relationship with IOP, some experts disagree that it is truly POAG. They believe it may be a totally separate condition. Either way, normal-tension glaucoma is extremely common.
Approximately 30% to 60% of people with POAG in white and Black populations have normal IOP. It’s even more common among people with POAG in Latino populations (approximately 80%) and Asian populations (47% to 92%).
Some people may develop NTG because their optic nerves have poor blood flow or are more structurally vulnerable to pressure. Another theory is that their eyes are less able to withstand normal pressure due to size, shape, wall thickness or corneal thickness.
It may also be due to an autoimmune issue or to higher sensitivity to typical, daily IOP fluctuations.
Secondary glaucoma
The optic nerve damage that occurs with a secondary glaucoma is the same as in a primary form. The difference between the two is that secondary glaucoma has causes that are distinct and identifiable.
This category is by far the more diverse of the two. Secondary glaucoma can be caused by many things, including underlying health conditions, eye disease and eye injuries. It can also be caused by medication side effects or eye surgery. It can be open-angle or angle-closure, and its different causes can lead to multiple types of glaucoma.
However, even though there are so many types, they only make up about 10% of all diagnosed glaucoma. Some of the more common types include:
Neovascular
Exfoliative
Pigmentary
Uveitic
Traumatic
Steroid-induced
Treatment-induced (caused by medical therapy)
The frequency of each type varies across different populations and parts of the world. But the neovascular, exfoliative, pigmentary and steroid-induced forms tend to be among the most common in general.
Neovascular glaucoma (NVG) – NVG is often a result of diabetic retinopathy or other causes of poor blood circulation of the eye. It develops when reduced blood flow to the retina triggers an overgrowth of abnormal blood vessels on the iris that eventually clogs the angle.
Exfoliative & pigmentary glaucoma – These are separate but similar forms caused by exfoliation syndrome (ES) and pigment dispersion syndrome (PDS). Accumulation of abnormal cellular dandruff (with ES) or flakes of iris pigment (with PDS) in the angle lead to high IOP and glaucoma.
Steroid-induced glaucoma – This type develops when steroid use (prescribed and OTC) causes both structural changes and increased debris deposits in the TM. Drainage resistance in the TM increases IOP and leads to glaucoma.
LEARN MORE about the types and causes of secondary glaucoma
Risk factors
Most forms of glaucoma have very similar risk factors, though there are a few differences. The biggest risk factor for all types of glaucoma is high intraocular pressure.
The risk factors that overlap for nearly all types of glaucoma include:
Having high IOP
Being over age 40
Having high blood pressure or heart disease
Having diabetes
Having a family history of any type of glaucoma
Having thin corneal tissue
Some risk factors are mainly associated with certain types of glaucoma. There are a few overlaps with these, as well:
Increased risk for POAG:
Having Black or Latin-American ancestry
Having myopia (nearsightedness)
Increased risk for PACG:
Having Asian, East Asian or Native Alaskan ancestry
Having anatomically larger lenses or thicker irises
Having hyperopia (farsightedness)
Being assigned female at birth
Increased risk for NTG:
Having anatomically larger optic discs
Having Asian or Latin-American ancestry
Having low blood pressure
Having migraines
Having Raynaud phenomenon
Having sleep apnea
Increased risk for secondary glaucoma:
Having diabetes
Using steroids
Having a previous eye injury or surgery
Having previous eye conditions, such as uveitis, infections and retinal detachment
When to see an eye doctor
Most people have no idea they have glaucoma until they experience significant changes to their vision. The damage that glaucoma causes initially affects areas of the visual field that the brain can "hide" for quite a long time. It doesn't become noticeable until this is no longer possible.
Because there are rarely any symptoms, comprehensive eye exams are the only way to catch the condition early. Glaucoma causes permanent loss of vision and, if left untreated, blindness. Early detection and treatment can stop its progression before there is enough vision loss to affect daily life.
Experts recommend yearly eye exams — not to be confused with vision screenings — for everyone beginning at age 6. People who are at higher risk for glaucoma may need to have more frequent eye exams.
Glaucoma. Johns Hopkins Medicine. Accessed April 2023.
Glaucoma. American Optometric Association. Accessed April 2023.
What is glaucoma? Glaucoma Research Foundation. Accessed April 2023.
What is glaucoma? In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Drainage system of the eye. World Glaucoma Association. Accessed April 2023.
Structure and function of the eyes. Merck Manual Consumer Version. September 2022.
Ciliary body. A.D.A.M. Medical Encyclopedia [Internet]. Ebix, Inc. September 2021.
Physiology, aqueous humor circulation. StatPearls [Internet]. January 2022.
Intraocular pressure. StatPearls [Internet]. July 2022.
Glaucoma and the importance of the eye's drainage system. BrightFocus Foundation. April 2018.
The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal Français d'Ophtalmologie. September 2020.
Open angle glaucoma. World Glaucoma Association. Accessed April 2023.
Primary open-angle glaucoma. Merck Manual Professional Version. September 2022.
Open angle glaucoma. StatPearls [Internet]. August 2022.
Angle-closure glaucoma. Merck Manual Professional Version. September 2022.
What is glaucoma? In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Ocular hypertension. World Glaucoma Association. Accessed April 2023.
Neuroanatomy, cranial nerve 2 (optic). StatPearls [Internet]. November 2022.
The quest to restore lost vision and cure glaucoma. Glaucoma Research Foundation. May 2022.
Lifestyle habits and glaucoma. EyeWiki. American Academy of Ophthalmology. January 2022.
Optic nerve cupping. Glaucoma Research Foundation. March 2022.
How did you get glaucoma? In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Low-tension glaucoma: an oxymoron in ophthalmology. Preventing Chronic Disease. January 2019.
Genetic variants associated with glaucomatous visual field loss in primary open-angle glaucoma. Scientific Reports. December 2022.
Glaucoma and eye pressure. National Eye Institute. March 2022.
Are there treatments other than lowering eye pressure? In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Extracellular matrix remodeling in the retina and optic nerve of a novel glaucoma mouse model. Biology. February 2021.
Wound healing: extracellular matrix. In Sabiston Textbook of Surgery, 21st Edition. Elsevier. 2022.
What is the extracellular matrix? MilliporeSigma. Accessed April 2023.
Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nature Communications. August 2018.
T cell-mediated autoimmunity in glaucoma neurodegeneration. Frontiers in Immunology. December 2021.
Glaucoma: Facts & figures. BrightFocus Foundation. October 2022.
Primary vs. secondary angle closure glaucoma. EyeWiki. American Academy of Ophthalmology. February 2023.
What is chronic angle-closure glaucoma? EyeSmart. American Academy of Ophthalmology. December 2021.
Angle-closure glaucoma. Merck Manual Professional Version. September 2022.
Angle closure glaucoma. World Glaucoma Association. Accessed April 2023.
Laser peripheral iridotomy: surgery for narrow-angle glaucoma. BrightFocus Foundation. July 2021.
The genetics of glaucoma. Glaucoma Research Foundation. March 2022.
Open-angle glaucoma: Epidemiology, clinical presentation, and diagnosis. UpToDate. September 2022.
Drug-induced acute angle-closure glaucoma: raising your index of suspicion. Biomedical Journal of Scientific & Technical Research. June 2021.
Primary Congenital Glaucoma. EyeWiki. American Academy of Ophthalmology. August 2022.
Childhood Glaucoma Research Network classification and epidemiology of childhood glaucoma. EyeWiki. American Academy of Ophthalmology. July 2022.
Juvenile glaucoma. StatPearls [Internet]. September 2022.
Normal-tension glaucoma. BrightFocus Foundation. August 2021.
Normal-tension glaucoma: questions and answers. Glaucoma Research Foundation. February 2022.
Latest developments in normal-pressure glaucoma: Diagnosis, epidemiology, genetics, etiology, causes and mechanisms to management. Asia-Pacific Journal of Ophthalmology. December 2019.
Secondary glaucoma. In Glaucoma: What every patient should know. Wilmer Eye Institute, Johns Hopkins School of Medicine. Accessed April 2023.
Secondary glaucomas. Glaucoma UK. Accessed April 2023.
Types of glaucoma. Dean McGee Eye Institute. Accessed April 2023.
Glaucoma eye health. BrightFocus Foundation. Accessed April 2023.
Primary angle-closure glaucoma: What to know. BrightFocus Foundation. August 2021.
Normal tension glaucoma. Wills Eye Hospital. Accessed April 2023.
Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness – a review. Restorative Neurology and Neuroscience. November 2018.
Comprehensive adult eye and vision examination, second edition. American Optometric Association. January 2023.
New AOA adult eye guideline offers 14 actions, provides fresh insight on exam frequency. American Optometric Association. March 2023.
Page published on Wednesday, February 27, 2019
Page updated on Tuesday, August 8, 2023
Medically reviewed on Thursday, April 27, 2023